-
Infrared Spectra of Small Insertion and Methylidene Complexes in Reactions of Laser-Ablated Palladium Atoms with Halomethanes
H.-G. Cho, L. Andrews, B. Vlaisavljevich and L. Gagliardi
Organometallics, 28 (24) (2009), p6871-6879


DOI:10.1021/om900750t | unige:4800 | Abstract | Article HTML | Article PDF
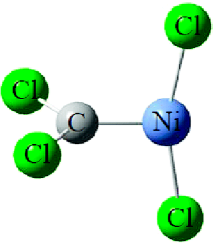
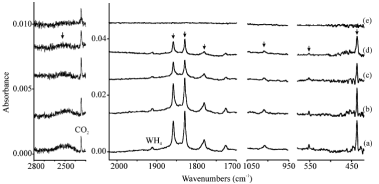
Palladium carbene complexes, CX2=PdX2, are prepared along with the insertion products, CX3âPdX, in reactions of laser-ablated Pd atoms with tetrahalomethanes and identified from matrix infrared spectra and density functional frequency calculations. The carbonâmetal bonds of the CCl2=PdCl2 and CClF=PdCl2 complexes are essentially double bonds with effective bond orders of 1.9, near those for the Pt and Ni analogues, as calculated by CASPT2 methods. On the other hand, only insertion complexes are generated from mono-, di-, and trihalomethane precursors. While the carbenes have staggered allene-type structures, many insertion complexes containing CâCl bonds reveal distinct bridged structures, which indicate effective coordination of Cl to the metal center.